How to Find Number of Protons
The nucleus of an atom contains both protons and. The number of protons in an isotope atom does not change but the number of neutrons does.

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets
Your mass number is the total number of neutrons and protons within the atom.

. The first thing you will need to do is find some information about. We can state the relationship between protons neutrons and mass. The mass number of an atom is calculated by adding the number of protons and neutrons.
Number of protons in 1 atom of hydrogen Atomic number 1. The atomic number is equal to the number of protons. Lithium is the 3rd element of the periodic table so its atomic number is 3.
You already know how to find the mass number of an isotope by adding the protons and neutrons. For neutral atoms the. So from figure 3 the number of electrons for chloride ion is 17 1 18.
Number of protons 11. Calculate the number of protons neutrons and electrons it contains. To find the number of protons electrons and neutrons in an atom just follow these easy steps.
Free and user-friendly Nernst Equation determines the reduction potential of a cell in a split of seconds along with the step by step solution guide. The letter A stands for it. To know how to find protons neutrons and electrons in an isotope let us consider the example of Chlorine isotopes Chlorine 35 17 Cl 35 and Chlorine 37 17 Cl 37 Chlorine 35.
Find Number Of Protons. Such as 8 C 9 C 10 C 11 C 12 C 13 C 14 C 15 C. Your atomic number is the amount of protons within the atom.
Step 1 - Gather Information. If the number of protons is given. The carbon atom has more than fifteen isotopes.
Number of protons in 3 hydrogen atoms 1 x 3 3. So to get the number of electrons you must add the size of charge to the atomic or proton number. The atomic number of a sodium atom is 11 and its mass number is 23.
Repeat for each element in the molecule then sum together all the. Of protons atomic number of neutrons mass number atomic number of electrons atomic number charge. It also explains the differe.
Number of protons in 1 molecule of ammonia 7 3. This chemistry video tutorial explains how to calculate the number of protons neutrons and electrons in an atom or in an ion. A N Z Calculations.
If an element is given find the atomic number of the element in the periodic table. Great lets apply the rules to some. Multiply the elements atomic number by the number of atoms of this kind in the molecule see Step 1.
The atomic number of an element is equal to the number of protons and electrons in that element.

How To Find The Number Of Protons Neutrons And Electrons
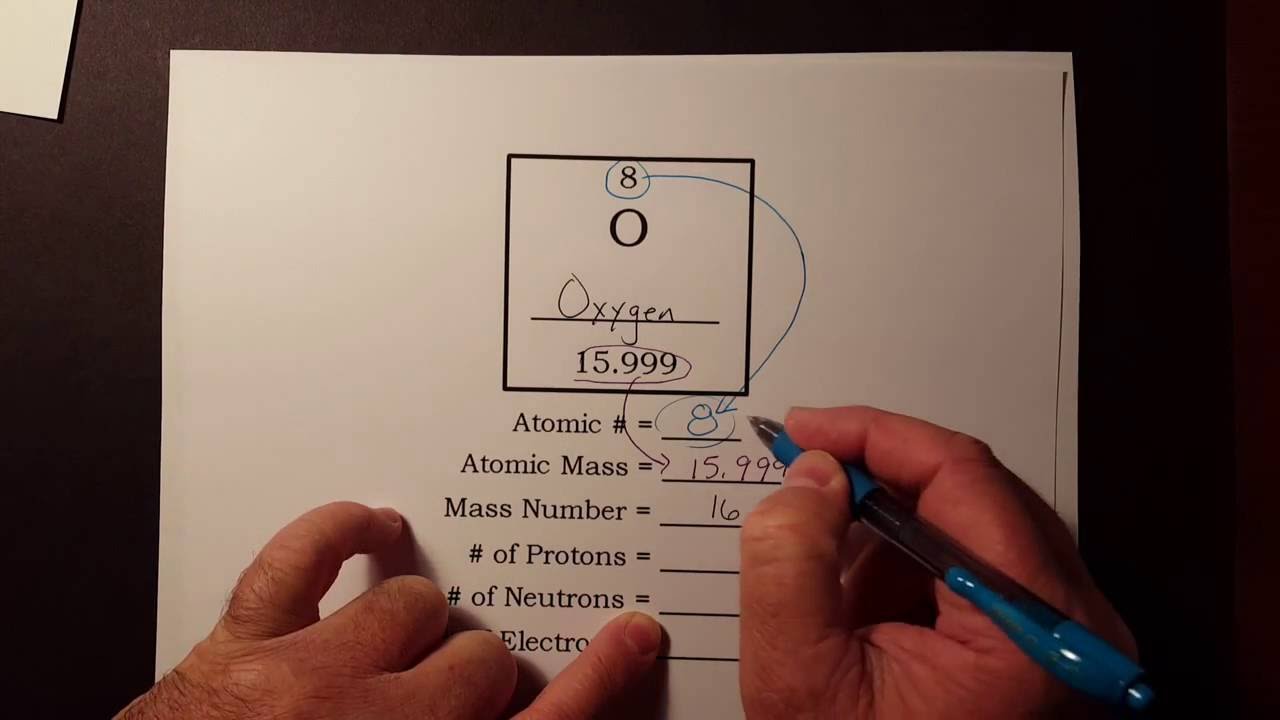
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Neutrons Protons Proton Neutron Electron

Finding Protons Neutrons And Electrons Through The Atomic Number And Neutrons By Mass Atomic Proton Neutron Electron Protons Neutrons



Comments
Post a Comment